Corneal blindness has affected more than 10 million lives on a global level. Cellular regeneration of corneal epithelium is a possible treatment option, prompting substantial research in the area. Human eye limbal fibroblasts have emerged as key cells in epithelial regeneration. They maintain the niche for corneal epithelial stem cells and repair tissue injury. Therefore, these cells have become a center for scientific investigation for developing novel therapeutics. This blog explores the biology, function, and therapeutic promise of primary human eye limbal fibroblasts in regenerative ophthalmology.
Limbal Fibroblasts
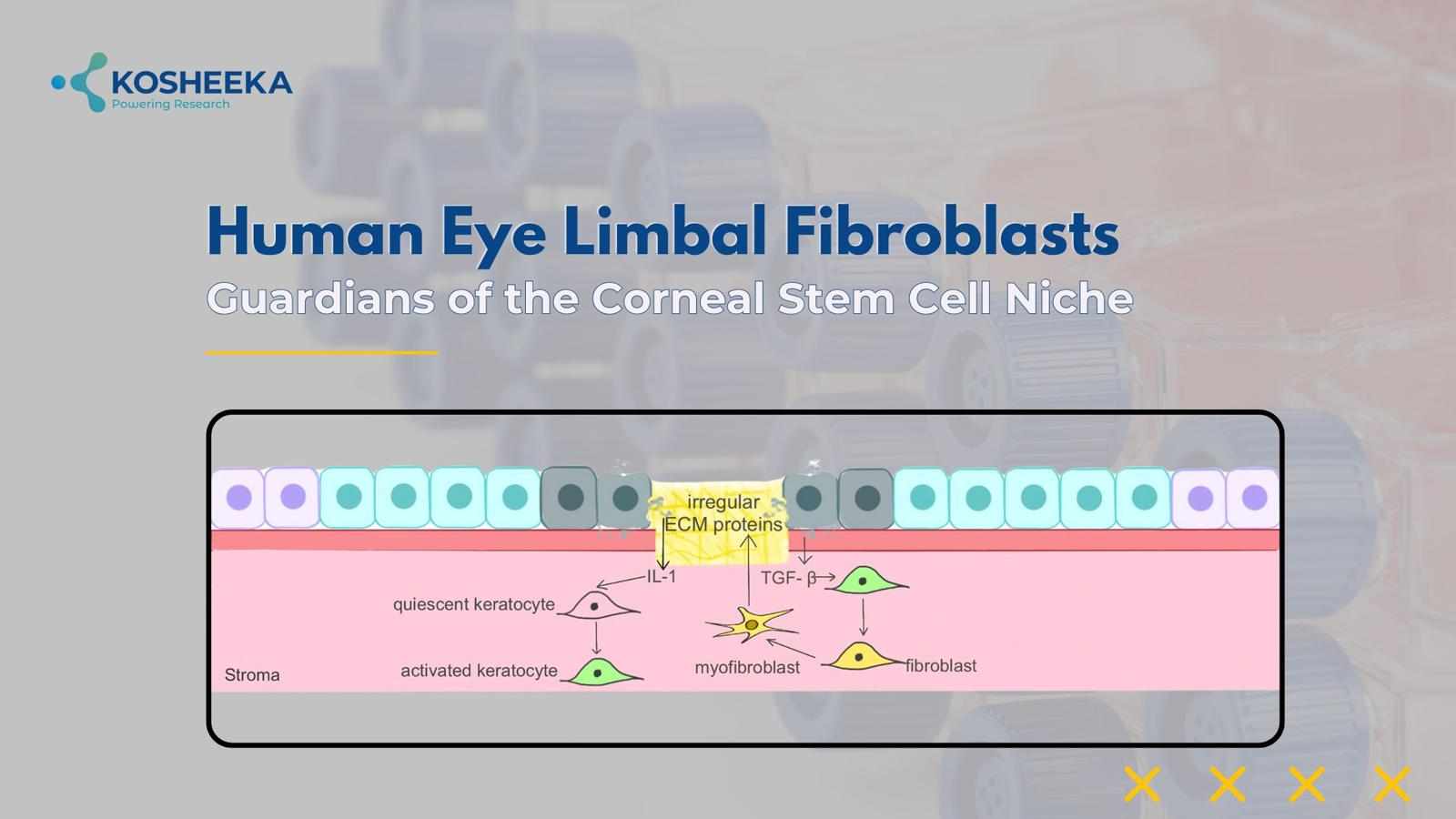
The cornea is the transparent covering on the eye surface comprising three layers: the outer epithelium, the middle stroma, and the inner endothelium. The stroma contains collagen-rich extracellular matrix (ECM) and keratocytes. The limbus region of the eye represents the peripheral region of the cornea near the conjunctiva. It has a unique microenvironment comprising epithelium, keratocytes, and blood vessels. Keratocytes are also referred to as stromal or limbal fibroblasts. These keratocytes transition to fibroblasts under the influence of transforming growth factor (TGF) and platelet-derived growth factor (PDGF), whereas the transition back to keratocytes occurs due to fibroblast growth factor (FGF) and retinoic acid. Keratocytes or limbal fibroblasts are joined by gap junctions to form a network. They also maintain an ECM that permits the penetration of optimal light into the eyes.
Function
Limbal or stromal fibroblasts originate from neural crest and exhibit characteristics similar to mesenchymal stem cells (MSCs). Just like MSCs, they also maintain the niche for limbal Stem Cells and express markers—CD106, CD105, CD90, CD154, CD166, CD71, and CD29. Owing to their contribution to the microenvironment, these cells are present in close vicinity to the stem cells. They also promote the proliferation of the limbal epithelial cell population during wound repair while also releasing chemokines to recruit progenitors to the injured site. Considering the similarities with MSCs, scientists have considered stromal fibroblasts to be multipotent in nature. Some of their subtypes have demonstrated differentiation into adipocytes, neurons, myocardiocytes, etc. Research has even suggested their ability to regenerate corneal epithelium.
Limbal Fibroblasts in Corneal Wound Healing
Corneal injury causes the apoptosis of stromal fibroblasts. If the matrix remains unharmed, then these cells proliferate and heal the injury. However, in cases of severe wounds, the lost matrix leads to the activation of the surrounding fibroblasts. They remodel the matrix while a small population of stromal fibroblasts forms myofibroblasts that confer contractility for healing corneal injury. Apoptosis of myofibroblasts is suggested to resolve the corneal injury. They also release ECM identical to the cornea and secrete antifibrotic and antiangiogenic factors such as IL-6, hepatocyte growth factor (HGF), and keratinocyte growth factor to promote tissue repair.
Limbal Fibroblasts in Research
Epithelial regeneration has prompted the culture of limbal epithelial cells. However, they differentiate in the in vitro environment through epithelial-to-mesenchymal transition. Gradually, the differentiated cells undergo senescence and decline in number. On the other hand, limbal fibroblast cells can gain stemness by mesenchymal-to-epithelial transition. Studies have shown that they can transdifferentiate into epithelial cells. However, the mechanism is still not clear, making them promising candidates for research and epithelial regeneration.
For the formation of epithelial grafts, co-culture models have been efficient for epithelial cells with fibroblasts. These systems have been using murine 3T3 fibroblasts; however, recent studies have shown that co-culture with stromal fibroblasts drives proper growth and differentiation of these cells within a short time span.
Limbal Fibroblasts in Disease
Epithelial stem cell deficiency is marked by the loss of limbal epithelial stem cells and their microenvironment. The deficiency causes epithelial vascularization, inflammation, and conjunctivalization, gradually increasing the corneal opacity, resulting in partial to complete visual loss. Xeroderma pigmentosum, chemical insult, injury, and graft vs. host disease are a few of the common reasons for the disorder. The therapy includes transplanting an epithelial stem cell graft. However, allogeneic transplants result in immune rejection, whereas autograft lacks stem cell availability in sufficient quantity. Their culture with MSCs yielded better outcomes. As compared to MSCs, stromal fibroblast cells contribute to the niche, causing reprogramming and the formation of epithelial sheets. Therefore, hinting at their therapeutic use.
The Concluding Statement
Limbal fibroblasts play a vital role in tissue homeostasis in the cornea. Their MSC-like properties have garnered attention from the scientific community. The studies have suggested better epithelial stem cell differentiation in the presence of stromal fibroblasts. These cells have been undermined for years, but the recent evidence indicates their indispensable contribution to the stem cell microenvironment and epithelial regeneration. More research will fully unlock their regenerative capabilities and mechanisms. Stromal fibroblasts represent a compelling target for future cell-based therapies in ophthalmology. As our understanding deepens, these versatile cells may become central to treating vision-threatening corneal disorders and advancing the field of regenerative eye care. Kosheeka provides the Human Eye Limbal Fibroblasts with assured quality to expedite such breakthroughs.





Write a comment ...